A degenerative disease, glaucoma is the leading cause of blindness in North America and Europe and is the second leading cause worldwide. It is most often associated with an increase of intraocular pressure (IOP). The most common form of the disease, primary open-angle glaucoma (POAG), is a sight-threatening condition caused by suboptimal ocular outflow leading to elevated IOP. While conventional surgical interventions such as aqueous shunts and trabeculectomy are effective in lowering IOP and are still widely used, they can be associated with numerous intraoperative and postoperative complications and safety issues.1,2 Consequently, surgeons are increasingly turning towards non-penetrating, bleb-free, minimally-invasive glaucoma surgery (MIGS) procedures. However, while MIGS procedures appear to be safer than conventional surgical interventions, they are not as clinically effective.3
Ab-interno Canaloplasty is a new MIGS procedure based on time-tested experience with Canaloplasty, a minimally invasive, restorative surgical procedure. Canaloplasty has been shown to be both safe and effective in more than 50 peer-reviewed clinical studies.4,5 It has also been shown to be equally effective as trabeculectomy.4,5
Offering the clinical efficacy of Canaloplasty via a simplified and much faster surgical approach, ab-interno Canaloplasty can be successfully combined with phacoemulsification in order to make best use of time in the operating room. Additionally, ab-interno Canaloplasty restores the function of the eye's natural outflow system without the need for a filtering bleb — offering an unprecedented level of efficacy and safety in the surgical treatment of glaucoma.

Unlike trabeculectomy, ab-interno Canaloplastydoes not produce a filtering bleb. Patients who undergo the procedure can continue normal day-to-day activities directly following treatment and require minimal post-operative follow-up.
The Ab-interno Canaloplasty Difference
Ab-interno Canaloplastyis the only MIGS procedure that successfully and comprehensively addresses all aspects of potential outflow resistance. Like traditional Canaloplasty, ab-interno Canaloplastytar gets the trabecular meshwork, Schlemm’s canal and collector channels – structures that control ocular outflow. It also follows the same dilation principles of traditional Canaloplasty, where precisely controlled delivery of Healon/Healon GV during withdrawal of the catheter allows the compressed tissue planes of the trabecular meshwork to separate, and any herniated inner wall tissue to withdraw from the collector channels. It is important to note that Canaloplasty ab-interno Canaloplasty are the only currently available procedures that address blockages in the collector channels.
MIGS procedures lower IOP by addressing different aspects of (rather than all aspects of) the ocular outflow system. For example, the Trabectome®, uses an electrosurgicalpulse to ablate the trabecular meshwork and inner wall of Schlemm's canal, while the iStent® works as a trabecular micro-bypass by allowing aqueous humor to flow directly from the anterior chamber into Schlemm's canal, thus circumventing the trabecular meshwork.6
In addition to addressing all aspects of ocular outflow, ab-interno Canaloplasty is also fast and easy to perform. The combined procedure (phacoemulsification and ab-interno Canaloplasty) is routinely performed in 10 to 15 minutes, with the ab-interno Canaloplasty aspect taking approximately five minutes to perform.
Clinical Results
Preliminary findings from a 70-eye case series by Mark J. Gallardo, MD (El Paso Eye Surgeons, PA) show reductions in IOP and in the number of medications similar to the results obtained with traditional Canaloplasty and trabeculectomy. Importantly, ab-interno Canaloplastymay also offer better clinical outcomes than any other currently available MIGS procedure.
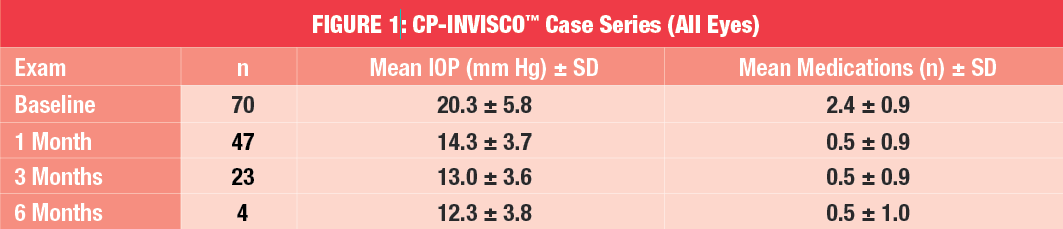
Mean preoperative IOP was 20.3 ± 5.8 mm Hg and the mean number of medications was 2.4 ± 0.9. At one, three and six months post-treatment, mean IOP was 14.3 ± 3.7 mm Hg, 13.0 ± 3.6 mm Hg and 12.3 ± 3.8 mm Hg, respectively, while the mean number of medications was 0.5 ± 0.9 at one and three months, and 0.5 ± 1.0 at six months post-treatment (refer to Figure 1).
Subgroup analyses were also performed including patients with (n=10) or without (n=48) previous surgery. The effect of ab-interno Canaloplasty on IOP and medication-use was also evaluated in pseudophakic patients (n=12).
In patients who had not undergone surgery prior to treatment, mean IOP improved from 18.8 ± 4.7 mm Hg at baseline to 13.9 ± 3.6 mm Hg at one month and 12.2± 1.9 mm Hg at three months post-treatment. Six-month data for three patients showed that mean IOP was 11.3± 4.0 mm Hg at this follow-up visit. The mean number of anti-glaucoma medications also reduced, from 2.2 ± 0.9 at baseline to 0.4 ± 0.7 and 0.2 ± 0.6 at three and six months post-treatment, respectively. Improvements in IOP and medication-use were also observed in patients who had undergone prior glaucoma surgeries. Mean IOP and mean medication-use improved from 25.8 ± 8.0 mm Hg and 3.0 ± 0.8 medications pre-treatment, to 15.3 ± 4.1 mm Hg and 1.0 ±1.5 medications at one month post-treatment and 18.3 ± 7.5 mm Hg and 2.3 ± 0.6 medications at three months post-treatment.
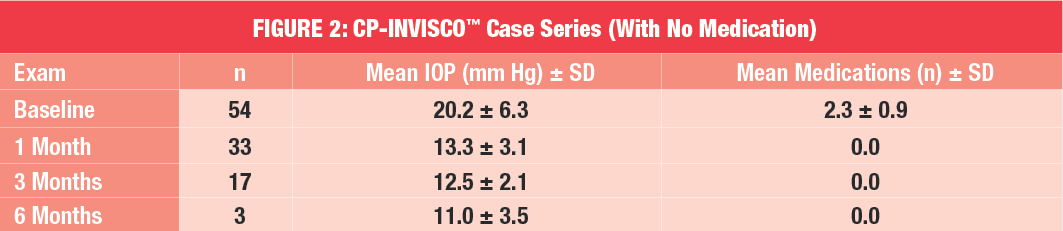
Furthermore, over 70 percent of patients were off medication entirely at one month and three months post-treatment (Refer to Figure 2). In pseudophakic patients, mean IOP improved from 21.8 ± 5.2 mm Hg at baseline to 15.1 ± 4.1 mm Hg at one month postoperative. Medication-use was also reduced, from 2.8.± 0.7 mm Hg at baseline to 0.8 ± 1.0 mm Hg at one month post-treatment. Case observation of ab-interno Canaloplasty also revealed that the safety profile of the procedure was similar to that of traditional Canaloplasty and the newer MIGS procedures.
Ab-interno Canaloplasty: MIGS Redefined
In summary, clinical evidence indicates that ab-interno Canaloplasty is safe and effective in mild-to-moderate POAG with similar IOP-lowering effects to tried and true traditional Canaloplasty. Unlike other MIGS procedures, ab-interno Canaloplasty ensures that all potential "blockages" in the ocular outflow pathway are addressed, including distal structures such as the collector channels, which have been shown to play a key role in blocking aqueous outflow in POAG eyes. Ab-interno Canaloplastyis also fast to perform and, unlike other currently available MIGS procedures, preserves tissue and does not require permanent placement of an implant in the eye. Furthermore, based on preliminary data it may, potentially, offer better clinical outcomes than any other currently available MIGS procedure.
Ab-interno Canaloplasty: Benefits at a Glance
1. Comprehensive: treats trabecular meshwork, Schlemm's canal and collector channels
2. Opens outflow system behind the trabecular meshwork, thus ensuring better aqueous outflow
3. No permanent implant or stent
4. On label — patient does not have to pay additionally out of pocket
5. Reimbursement is higher than current MIGS procedures
6. Patient selection criteria are similar to current MIGS procedures
7. Easy to explain to the patient, for example: "I am going to perform circumferential angioplasty in your eye to reduce pressure" or, "We want to avoid cutting tissue or inserting devices; so let's try CP-INVISCO™ first."
Ab-interno Canaloplasty: Key Treatment Steps
STEP 1
Following cataract surgery, inject Miostat into the anterior chamber, followed by dispersive viscoelastic. Create side port for the iTrackTM microcatheter approximately 1 1/2 clock hours away from 3 o’clock (right eye) or 9 o’clock (left eye) position. Insert the primed iTrack™ into the anterior chamber.
.png)
STEP 2
Entering at the temporal location, create a small horizontal incision ~1mm-wide in the trabecular meshwork. (Note the position of iTrack™ above the modified Cystatome in the image pictured to the right.)
.png)
STEP 3
Using MST retina forceps, feed iTrack™ into Schlemm's canal. Align iTrackTM flush to the trabecular meshwork. Follow progress by observing the positional red light. Advance the tip of iTrack 360° to initial incision site.
.png)
STEP 4
Slowly withdraw iTrack™ while steadily injecting viscoelastic. Once complete, remove hemorrhage from the incision site and all dispersive viscoelastic from the anterior chamber before re-pressurizing with BSS to 20 mmHg.
.png)
References:
1. Rulli E; Biagioli E, Riva I, Gambirasio G, De Simone I, Floriani I, Quaranta L. Efficacy and safety of trabeculectomyvsNonpenetrating Surgical Procedures: A Systematic Review. JAMA Ophthalmol. 2013;131(12):1573-1582. doi:10.1001/jamaophthalmol.2013.5059
2. Zahid S, Musch DC, Niziol LM, Lichter PR; Collaborative Initial Glaucoma Treatment Study Group. Risk of endophthalmitis and other long-term complications of trabeculectomy in the Collaborative Initial Glaucoma Treatment Study (CIGTS). Am J Ophthalmol. 2013 Apr;155(4):674-680, 680.e1. doi: 10.1016/j.ajo.2012.10.017. Epub 2012 Dec 13.
3. Brandão LM, Grieshaber MC. Update on minimally invasive glaucoma surgery (MIGS) and new implants. J Ophthalmol. 2013:705915.
4. Brüggemann A, Despouy JT, Wegent A, Müller M. Intraindividual comparison of Canaloplasty versus trabeculectomy with mitomycin C in a single-surgeon series. J Glaucoma. 2013; 22(7):577-83.
5. Klink T, Sauer J, Körber NJ, et al. Quality of life following glaucoma surgery: canaloplasty versus trabeculectomy. ClinOphthalmol. 2014;18;9:7-16.
6. Nichamin LD. GlaukosiStent Trabecular Micro-Bypass. Middle East Afr J Ophthalmol. 2009; 16(3):138-40.

